GAMP 5: Implementation & Operation of GxP Compliant Clinical System | ISPE | International Society for Pharmaceutical Engineering

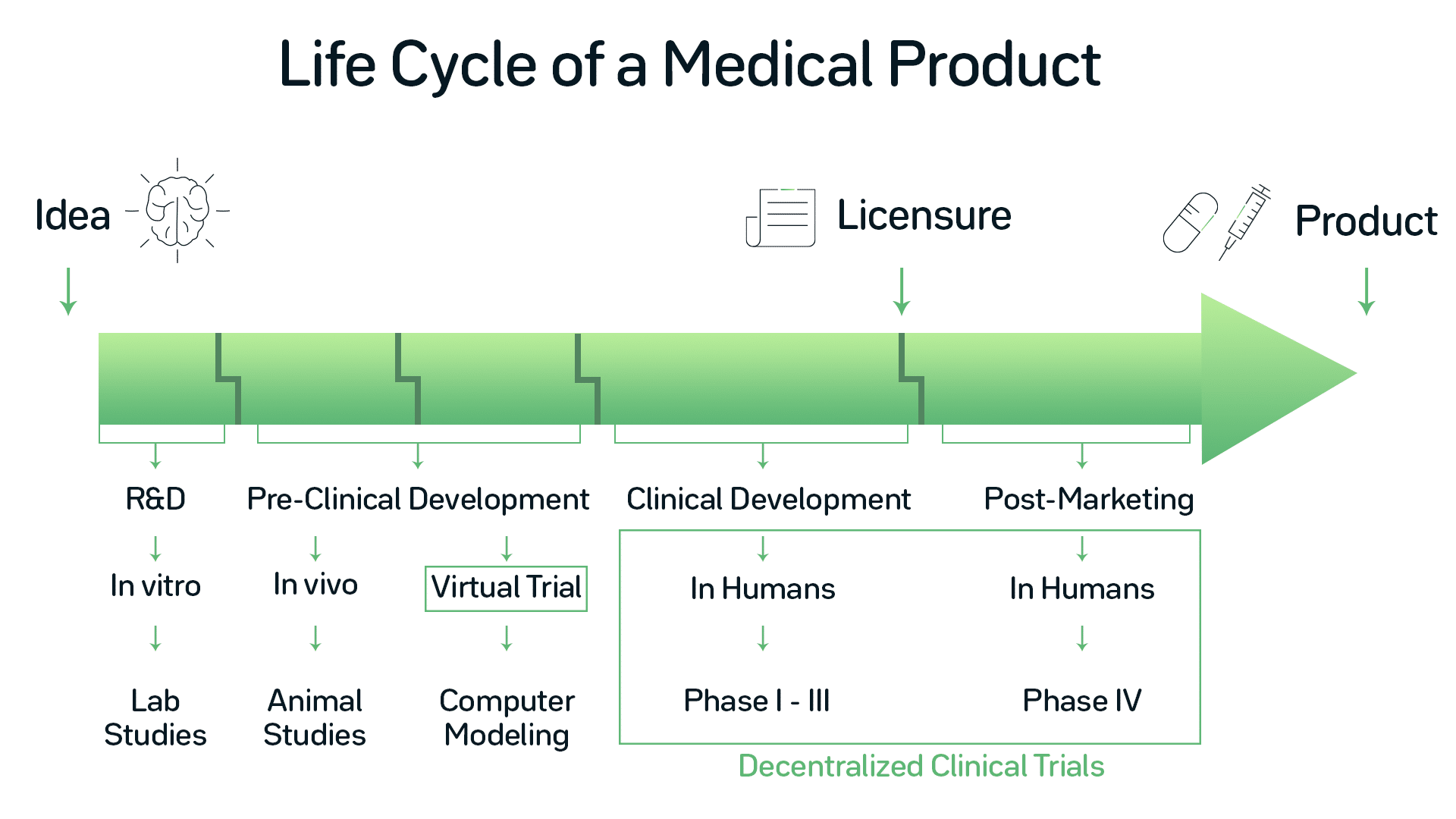
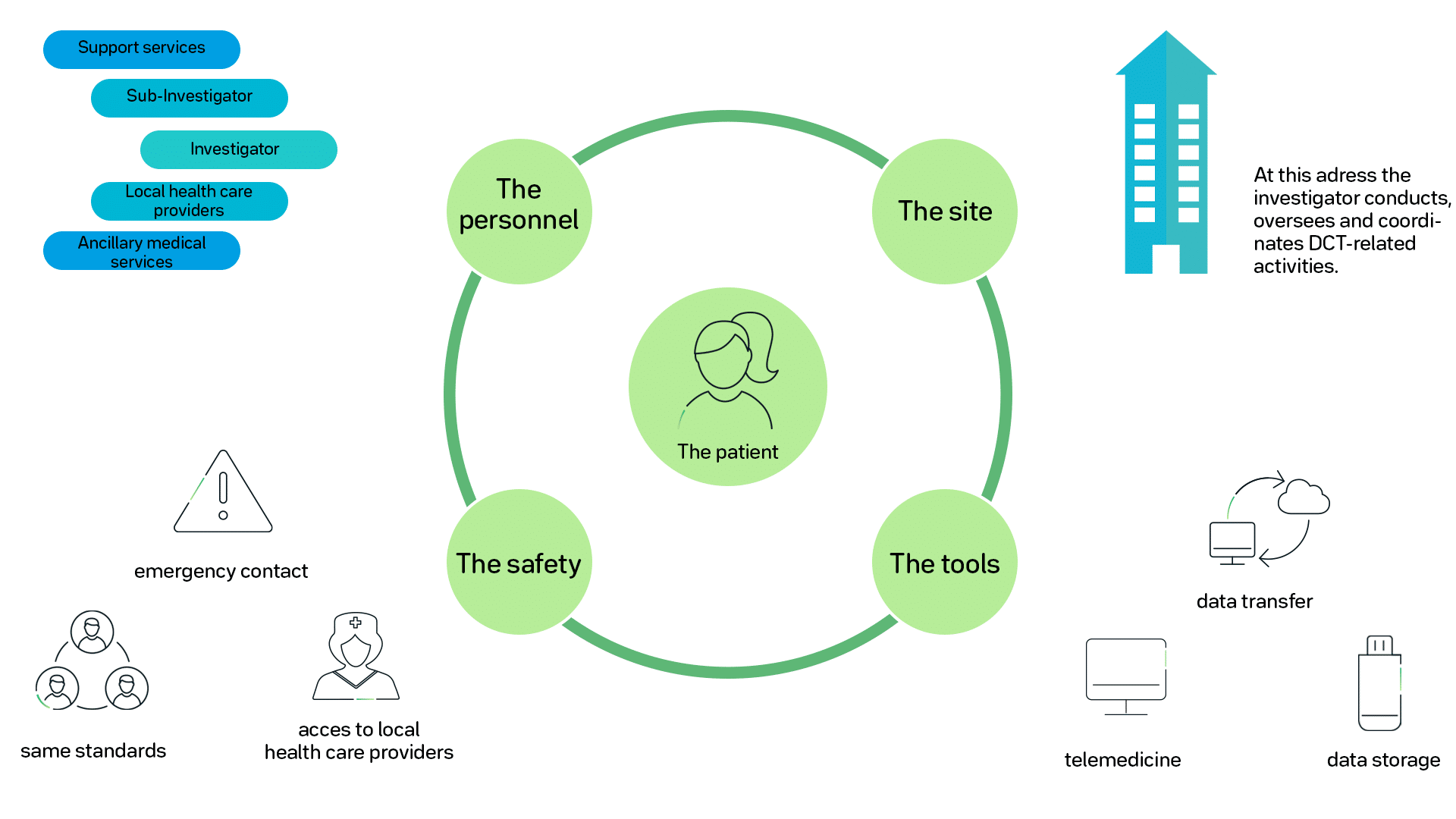
Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - ScienceDirect

Clinical trials: EMA launches public consultation on draft guideline for using computerized systems and electronic data - Portolano Cavallo

Performance at different clinical trial sites Discussion This is one of... | Download Scientific Diagram

Data Integrity in Global Clinical Trials: Discussions From Joint US Food and Drug Administration and UK Medicines and Healthcare Products Regulatory Agency Good Clinical Practice Workshop - Khin - 2020 - Clinical